Harnessing the power of the body’s own cells to give people hope.
When vascular-related conditions don’t respond to treatment, or conventional therapies aren’t an option, they can be devastating. They compromise people’s quality of life, drain them of hope for the future and may cause death while costing the healthcare system billions of dollars.
At VESSL, we’re on a mission to change all that…and change lives, too.
We’re using cell- and gene-based proprietary technologies along with the body’s own healing mechanisms to develop solutions for vascular-related conditions that are effective and faster to recover from. Our breakthrough solutions are aimed for people suffering from advanced peripheral arterial disease, problems with their dialysis access sites and many other conditions.
We’re not just giving them new options. We’re giving them new hope.
And as fascinating as our work is, that’s what we look forward to every day.
Technology
Technology has changed a lot in fifteen years.
What motivates us has not.
VESSL’s core technology is enabling us to develop entirely new, life-changing treatments for a wide range of blood-vessel-related disorders.
We combine a patient’s own endothelial cells with specific genes and other synergistic cell types to create groundbreaking therapies for patients with peripheral arterial disease, coronary artery disease, end-stage renal disease, liver cirrhosis and diabetic retinopathy – patients for whom conventional treatments have failed or are unsuitable.
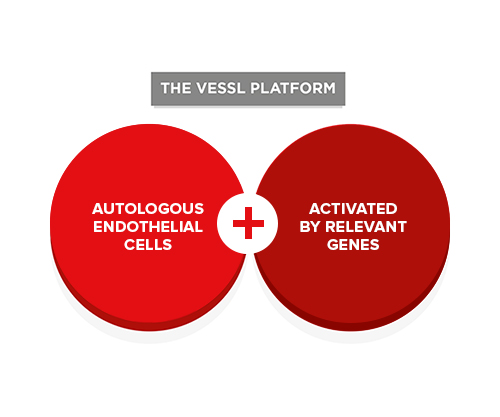
Our therapies are derived from the patient’s own cells isolated from a short vein segment taken from their arm under local anesthesia. These cells are activated by the insertion of specific genes and are then re-introduced to the patient.
Our pioneering approach enables the secretion of multiple factors by the activated autologous cells, closely mimicking the natural process of blood vessel formation and growth.
VESSL’s leading solutions are expected to improve blood flow through the proliferation of collateral blood vessels or by a modified synthetic graft with greater biocompatibility.
We’ve been immersed in developing these treatments for fifteen years. It’s amazing how technology has changed in that time. And it’s equally amazing what it enables us to do today.
Solutions
New therapies that are giving patients
a whole new outlook on life.
MGA for PAD
Peripheral artery disease (PAD) is a systemic atherosclerotic disease. It afflicts 8 million Americans and an estimated total of 25 million patients in the developed world. It is associated with diabetes, obesity, hypertension, smoking and high cholesterol levels.
PAD is characterized by the narrowing or occlusion of arteries in the leg, resulting in inadequate blood flow to the leg muscles. This may cause pain when a patient engages in physical activity, but that pain will usually respond to rest. The situation could deteriorate, however, and progress to critical limb ischemia (CLI) with its attendant rest pain, ulcers that won’t heal or gangrene that may lead to limb amputation.
CLI, the most severe form of PAD, develops in about 4% of PAD patients. Currently, there are about 1 million patients in the developed world, with 200,000-300,000 of them either being unsuitable for or unresponsive to conventional therapies such as bypass surgery or balloons and stents. Their quality of life has been described as similar to that of a patient with terminal-stage cancer, and many will eventually have to undergo amputation or will die.
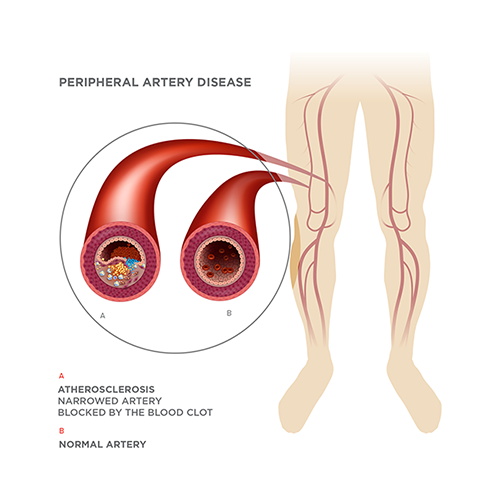
One of our primary solutions, MultiGeneAngio (MGA), is designed to offer hope to people with no other options.
Using blood vessel cells isolated from a patient’s own body and into which we insert specific genes, MGA harnesses the body’s own mechanisms to grow natural bypasses. By enhancing the formation of collateral arteries, MGA significantly increases blood flow to the oxygen-starved muscles.
MGA is expected to dramatically improve a patient’s quality of life, improve healing of ulcers, reduce amputations, minimize hospital stays and procedures and decrease the enormous costs associated with CLI.
To date, we have completed pre-clinical experiments and two phase I/IIa human trials of MGA in PAD patients at the University of Michigan, the University of Pennsylvania and 7 medical centers in Israel. Thirty-five patients have been treated, with no safety issues and promising early indications of efficacy.
MGG for ESRD
About 50% of all end-stage renal disease (ESRD) patients are maintained by hemodialysis, undergoing mechanical blood cleaning several times a week for the rest of their life (or until a kidney transplant becomes available). More than 400,000 patients rely on this therapy in the US and a total of 1.25 million patients rely on it in the developed world. The economic burden of managing vascular access dysfunction is enormous and includes recurring surgical procedures required for keeping the access site open. The number of ESRD patients is projected to keep growing due to the availability of fewer donor kidneys and the increasing prevalence of diabetes and hypertension.
Creating and maintaining a suitable arterio-venous access site is one of the most challenging and expensive aspects of hemodialysis treatment and frequently leads to hospitalization. Approximately 25% of all hospital stays for ESRD patients are related to problems with vascular access.
The preferred form of access is called an arterio-venous fistula. To create one, a surgeon connects a vein to an artery (typically in the arm), resulting in a high-flow circuit suitable for dialysis.
In 20% of these cases, poor vasculature requires the use of a synthetic graft in order to create an access site. The problem with synthetic grafts is that they have a lifespan of only two years before a replacement or correction is needed, and about half of them fail within 6 months.
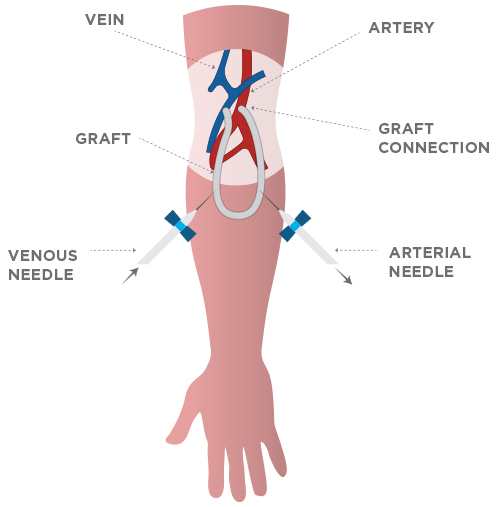
VESSL’s MultiGeneGraft (MGG) is a biosynthetic vascular graft lined with a patient’s own endothelial cells which are modified by specific genes. Lining the graft with these cells provides a clot-resistant vessel that’s expected to outlast and outperform synthetic products currently in use.
By improving compatibility and reducing complications, MGG will reduce interventions, hospitalization time and the number of required surgeries. Pre-clinical trials demonstrated successful adhesion of the endothelial cells to the graft material and a reduction in short- and long-term failure rates.
The FDA has granted IND for MGG, and a phase-I study in 10 hemodialysis patients is planned.
Pipeline
We have a bright future ahead of us. More importantly, so do patients.
Additional solutions using our technological platform are currently in different pre-clinical stages. These include:
- MultiGeneAngio (MGA) for treatment of coronary artery diseases (CAD)
- MultiGeneLiver (MGL) for liver regeneration in patients with liver cirrhosis
- MultiGeneEye (MGE) for treatment of diabetic retinopathy
Team
We’re driven by the science of caring.
The VESSL Management Team

Benny Zeevi, MD
Chairman of the Board
Dr. Benny Zeevi is a Managing General Partner at Tel Aviv Venture Partners/DFJ Tamir Fishman Ventures and RunYoung Capital and leads the funds Life Science investments.
Dr. Zeevi is deeply involved in the Israeli life sciences and digital health industries. He was the Co-Chairman of the IATI – Israel’s largest non-profit umbrella organization for the High-Tech and Life Science sectors and wrote the first comprehensive report about the Israeli life sciences industry. Dr. Zeevi is the Founder and Co-Director of an Executive Program for Biotechnology and Medical Device Entrepreneurs and Managers and serves on the advisory board of the MBA in Management of Technology, Innovation & Entrepreneurship, Faculty of Management at Tel Aviv University.
Dr. Zeevi serves as the chairman of the board, a board member and advisory board member of 9 life sciences companies in different subsectors.
Dr. Zeevi is a physician who specialized in interventional pediatric cardiology. He holds an MD degree (Magna Cum Laude) from the Sackler School of Medicine, Tel Aviv University and has authored over 60 peer-reviewed scientific articles, in addition to various chapters in textbooks and invited reviews and editorials in pediatric cardiology journals.

Prof. Moshe Flugelman, MD
Founder and Co-CEO
Prof. Flugelman leads the scientific and medical activities of VESSL. He specializes in interventional cardiology and vascular biology. Prof. Flugelman is the former Director of the Department of Cardiovascular Medicine at the Lady Davis Carmel Medical Center, Haifa, Israel, and a Professor of Medicine at the Technion, Israel Institute of Technology. Prof. Flugelman has authored over 150 peer-reviewed scientific articles.
Prof. Flugelman was a visiting Scientist at the National Heart, Lung & Blood Institute of the National Institutes of Health (NIH) in Bethesda, Maryland and was a fellow in experimental angioplasty at Washington Hospital Center, Washington, DC. While at the NIH, Prof. Flugelman worked with leading experts in vascular gene therapy. He has broad experience in the fields of experimental cardiology and vascular biology.
Prof. Flugelman earned his Medical degree at the Technion – Israel Institute of Technology and completed his cardiology training at the Hadassah Hebrew University Medical Center in Jerusalem.

Ranny Yaffe
Co-CEO
Mr. Ranny Yaffe is responsible for the business, financial and fiscal management of VESSL. He provides leadership and coordination in the business planning, logistical, accounting and legal efforts of the company, and he is responsible for optimizing its day-to-day operations.
Mr. Yaffe joined VESSL in 2004 after serving as Business Development Manager for several life science companies.
He holds a B.Sc. in Industrial Engineering and Management and an MBA with distinction, both earned at the Technion – Israel Institute of Technology.
Mr. Yaffe is also one of the founders of RemiWise Oncology, a drug development company focusing on novel therapies for refractory cancers.

Marina Hutoran, PhD
Director of Product Development
Dr. Marina Hutoran oversees product development and technology transfer of VESSL’s products. She previously led and supervised the technology transfer of VESSL’s manufacturing process in the US and Israel before and during the clinical trials.
Dr. Hutoran held leadership roles in R&D and IP management at NextFerm Technologies for 5 years before resuming her leadership role at VESSL.
Dr. Hutoran holds a PhD in Virology from the Hebrew University in Jerusalem.
Our Board of Directors
Roger Lu
Founder and Chairman, RunYoung Capital
Mr. Roger Lu has over 20 years of experience in corporate and financial management. He is a veteran of cross-border business with Israel and North America and has been responsible for many investments over the years.
Mr. Lu joined Sailing in 2018 as General Manager of Sailing Link Capital Management Company. Prior to that he was with PTL where he provided investment and advisory services to the Israeli market, participated in the completion of more than 20 projects with foreign investment and the establishment of a $1 billion joint venture between Israel’s second largest fund and China Everbright Bank Fund to introduce Israeli projects to the Chinese market. Mr. Lu also led the completion of five projects with Chinese investment in Israeli companies; three in the healthcare sector, one in water treatment and one in the internet sector.
Reuven Ben Menachem
Founder and former CEO of Fundtech
Mr. Reuven Ben Menachem is the Founder and former CEO of Fundtech, a leading global provider of software to the financial services sector.
Mr. Ben Menachem led Fundtech to a successful IPO in 1998 and raised $100 million in a secondary offering in 1999. He sold Fundtech to GTCR (a Chicago-based private equity firm) in 2011, and in 2015 Fundtech was sold to a strategic buyer for $1.25 billion.
Prior to there, Mr. Ben Menachem was employed at Logic Data Architects, as a Technical Director and Product Manager. From January 1984 to June 1986, he served as a Director of Banking Systems at Manof Communications Systems. Prior to this role, he served as a Senior Programmer and Analyst in the Israeli Air Force.
Mr. Ben Menachem studied for a B.A. in Business at Haifa University.
Prof. Moshe Flugelman, MD
Founder and Co-CEO, VESSL Therapeutics Ltd.
Prof. Flugelman leads the scientific and medical activities of VESSL. He specializes in interventional cardiology and vascular biology. Prof. Flugelman is the former Director of the Department of Cardiovascular Medicine at the Lady Davis Carmel Medical Center, Haifa, Israel, and a Professor of Medicine at the Technion, Israel Institute of Technology. Prof. Flugelman has authored over 150 peer-reviewed scientific articles.
Prof. Flugelman was a visiting Scientist at the National Heart, Lung & Blood Institute of the National Institutes of Health (NIH) in Bethesda, Maryland and was a fellow in experimental angioplasty at Washington Hospital Center, Washington, DC. While at the NIH, Prof. Flugelman worked with leading experts in vascular gene therapy. He has broad experience in the fields of experimental cardiology and vascular biology.
Prof. Flugelman earned his Medical degree at the Technion – Israel Institute of Technology and completed his cardiology training at the Hadassah Hebrew University Medical Center in Jerusalem.
Leigh Chen
Chairman and Founding Partner, TCC
Mr. Leigh Chen is the Chairman and Founding Partner of Tong Ce Capital (TCC), a private equity fund.
Prior to founding TCC in 2013, Mr. Chen was an Executive Director at Nomura International (HK) and a Vice President at Lehman Brothers Asia.
Our Scientific Advisory Board
Prof. Aaron J. Ciechanover, MD, DSc
2004 Nobel Prize laureate in Chemistry and distinguished Research Professor, Technion – Israel Institute of Technology.
Prof. Renu Virmani, MD
President and Medical Director, CVPath, International Registry of Pathology (Gaithersburg, MD, USA).
Clinical Professor, Department of Pathology, at five leading US universities.
Aharon Schwartz, PhD
Former Vice President of Strategic Business Planning and New Ventures at Teva Pharmaceutical Industries Ltd.
Prof. Paul Feigin, PhD
Professor of Statistics and Senior Executive Vice President of the Technion – Israel Institute of Technology.
Prof. Yosef Shaul, PhD
Department of Molecular Genetics and Virology, Weizmann Institute of Science (Rehovot, Israel).
News
VESSL Therapeutics Ltd. Closed $16m Funding Round Led by RunYoung Capital, to advance its cell and gene therapy regenerative medicine platform for patients with peripheral artery disease and dialysis access.
Haifa, Israel, Shanghai, China April 13th, 2021 – VESSL Therapeutics, a leader in regenerative medicine solutions based on proprietary cell and gene technology, announced the closing of a $16M series AA round of financing led by RunYoung Capital. Proceeds from the financing will be used to advance the development of the company’s two products and support their regulatory approval.
Led by Prof. Moshe Flugelman since its founding in 2000, VESSL Therapeutics is a biotechnology company that has developed a novel technology based on cell therapy for the treatment of vascular diseases in patients suffering from peripheral artery disease (MultiGeneAngio – MGA) and MultiGeneGraft (MGG) technology for dialysis access. treatments incorporate the patient’s own vascular cells.
Following on the successful completion of two Phase I/IIA clinical trials with 35 patients in the US and Israel under IND, the funding will facilitate conducting Phase IIB for the MGA product in patients with peripheral arterial disease and Phase IA for the MGG product for patients undergoing hemodialysis to support regulatory approval in certain territories.
Therapeutic angiogenesis is a method of improving blood flow by growing new blood vessels in areas of the body where arteries are blocked. The process is based on the use of genetic engineering using special angiogenic genes.
“VESSL Therapeutics technology and products are addressing the unmet need of no – option patients with peripheral vascular diseases. The completion of this financing round is an important milestone for VESSL Therapeutics and will accelerate the development of the company’s innovative medical and technological vision” said Prof. Moshe Flugelman, Founder and Co-CEO of VESSL Therapeutics.
“We are extremely pleased to have VESSL Therapeutics as our first-ever biotechnology investment in Israel, as it represents the pivotal role Israeli companies play in global life sciences innovation. VESSL Therapeutics is developing a very unique and potentially disruptive technology, based on advanced science and promising initial clinical results in patients, that should greatly advance the field of regenerative medicine I am positive that patients both in China, and around the world will benefit greatly from VESSL Therapeutics’ products” said Roger Lu, Chairman/Founder of RunYoung Capital. We have confidence in the leadership of Prof. Flugelman and Ranny Yaffe, the co-CEO’s of VESSL Therapeutics, to successfully bring its technology to the clinic, and make VESSL a global leader in regenerative medicine.
Dr. Benny Zeevi, Chairman of the Board of Directors added “VESSL Therapeutics technology provides the potential for breakthroughs in this area and represent a significant market opportunity. The scientific rigor behind VESSL Therapeutics technology is well recognized by leading scientists, and medical opinion leaders from all over the world. We are enthusiastic about having Roger Lu CEO and Lei Chen, Managing Partner of RunYoung Capital joining our board of directors”.
Contact us
VESSL Therapeutics Ltd.
Colab Square Facility
6 HaYozma Street
Yokneam Illit 2069203
Israel
Get in touch